COVID-19 Treatments, Therapeutics &
Test to Treat Information
Information on this page is a general overview of therapeutic treatments. In no way is the following information meant to replace or override the medical advice received from a licensed physician. Always check with your healthcare provider for a professional assessment on treatments that are right for you.
Tested positive for COVID-19? Get FREE Medication right away!
COVID-19 medications can prevent hospitalization and death. To access COVID-19 treatments:
- Talk to your health care provider
- Visit a Test to Treat location near you
- If you cannot access a health care provider within 24 hours, speak to someone over the phone or video call for free
- Call 833-686-5051
- Or make an appointment at sesamecare.com/covidca
Visit this link to learn more about what to do if you test positive for COVID-19.
About Treatments and Therapeutics
There are now free and effective COVID-19 treatments available for you! If you test positive for COVID-19, treatments can prevent you from needing to go to the hospital. Most treatments need to be started within 5 days of getting symptoms. As soon as you notice symptoms, find the nearest Test to Treat Site or make a virtual appointment with Sesame Care.
DON’T WAIT! Get tested and talk to a health care provider about your treatment options today!
Test to Treat and Telehealth Resources
If you have tested positive for COVID-19, consider starting treatment right away. COVID-19 treatments are safe, free, and highly effective! Most adults are eligible to receive these medications that have been proven to reduce risk of severe illness due to to COVID-19. To access COVID-19 treatments:
- Talk to your health care provider
- Visit a Test to Treat location near you
- If you cannot access a health care provider within 24 hours, speak to someone over the phone or video call for free
- Call (833) 686-5051
- Or make an appointment at sesamecare.com/covidca
COVID-19 medications must be taken within 5-7 days of symptoms starting to be effective. Visit this link to learn more about what to do if you test positive for COVID-19.
How do I know if qualify for treatments?
When you go through one of the three options listed above you will be thoroughly assessed from a trained health care provider. They will assess your current condition, ask you some questions about your medical history and review any current medications you are taking. Once they have completed the exam they will decide which, if any, COVID-19 Treatment is right for you.
What if I qualify for treatments and do not have any or full coverage insurance?
Most COVID-19 Treatments are available at no cost to you, as the patient! These treatments are proven to be safe and effective in preventing hospitalization and death. All treatments need to be given early in your COVID-19 diagnosis, so don’t forget to contact a health care provider as soon as you test positive.
Telehealth services provided by the California Department of Public Health are designed to support uninsured and underinsured individuals who cannot connect with a healthcare provider within 24 hours of receiving a positive test results.
What if I have health insurance?
If you are insured, try to reach your healthcare provider first. If you have difficulty doing so within 24 hours of your positive results, you may schedule a telehealth appointment by calling 833-686-5051 or visiting sesamecare.com/covidca. You may also visit a Test to Treat location near you.
What is Test to Treat?
Test to treat is a federal program initiative to begin bridging the gap between patients testing positive for COVID-19 and receiving oral antiviral medications. This program is intended to provide one central location for a patient to get tested, evaluated after their diagnosis, and receive oral antiviral medication should they fit the EUA requirements for receiving the medication.
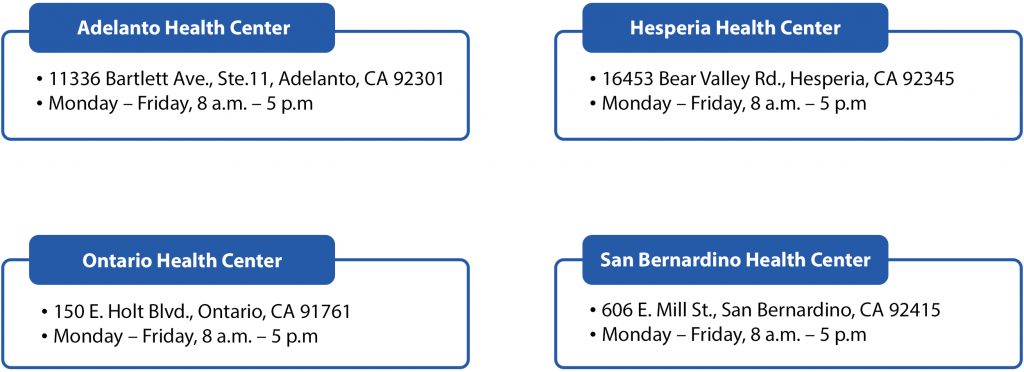
Where are the San Bernardino Department of Public Health (SBDPH) COVID-19 Test to Treat locations? (Including days of operation and hours)?
The health centers below are providing test to treat services to assigned IEHP and uninsured patients following California Department of Health Care Services (DHCS) guidelines. To schedule an appointment at one of San Bernardino County’s Public Health Clinics please call 1(800) 722-4777.
OptumServe Test to Treat
The SMARTER Plan is the next phase of California’s COVID-19 response. Included in this plan is providing opportunity for testing and treatments to patients regardless of insurance status or ability to pay. In an effort to have equitable access to end to end care for COVID-19, California Department of Public Health (CDPH) has partnered with OptumServe to open additional Test to Treat Sites across San Bernardino County.
Make an appointment at an OptumServe site near you. Use the Test to Treat Locator to ensure the site is offering this service.
If you have any questions, please call the OptumServe call center at (866) 284-8788.
How do I find a Test to Treat Site?
To find Test to Treat locations near you, view COVID-19 Test to Treat Locator.
You can call 1 (800) 232-0233 to get additional support in English, Spanish and 150+ languages.
Available 7 days a week from 8 a.m. to midnight EST
The Disability Information and Access Line is available to help people with disabilities access services via phone at (888) 677-1199 or email DIAL@usaginganddisability.org.
Available Monday – Friday from 9 a.m. – 8 p.m. ET
How does accessing COVID-19 treatments work?
Each patient will need a positive COVID-19 test, which can be administered on site at the Test to Treat facility or brought in by the patient for assessment. The patient will then need to be evaluated by a healthcare provider on site who can provide a prescription for the appropriate treatment.
What COVID-19 treatments are available?
Paxlovid: Can be prescribed to positive COVID-19 patients 12 years of age and older.
Molnupiravir: Can be prescribed to positive COVID-19 patients 18 years of age and older.
Make sure you follow directions provided by your healthcare provider or pharmacist.
When will Test to Treat be available?
Test to treat is available now! To find a location near you use the Test to Treat Locator. A call center is also available at 1-800-232-0233 (TTY 1-888-720-7489) to get help in English, Spanish, and more than 150 other languages – 8:00 a.m. to midnight ET, 7 days a week. The Disability Information and Access Line (DIAL) is also available to specifically help people with disabilities access services. To get help, call 1-888-677-1199, Monday-Friday from 9:00 a.m. to 8:00 p.m. ET or email DIAL@usaginganddisability.org.
If I have questions, who do I call or email? Is there a website for reference?
- For more information please refer to the Test to Treat Fact Sheet.
- San Bernardino County COVID Call Center 909-387-3911
- CDPH COVID-19 Treatments
*If you or a loved one, are experiencing ANY of the symptoms listed below call 911 or go to the nearest emergency room IMMEDIATELY.*
- Trouble breathing
- Persistent Pain/ Pressure in the chest
- New confusion
- Inability to wake or stay awake
- Pale, gray, blue or discolored skin, lips or nail beds (depending on skin tone)
Treatment Facilities
Therapeutic treatments are shipped countywide. Patients should consult with their respective physician or care provider before receiving COVID-19 treatments.
The link below will show you all facilities that have received COVID-19 therapeutics. However, patients should not contact locations directly. Always consult with your physician for eligibility to receive any COVID-19 Treatments.
Oral Antivirals
- Pfizer’s Paxlovid
- Merck’s Lagevrio (molnupiravir)


